Share
If 5.60 mol of a substance releases 14.9 kJ when it decomposes, what is ΔH for the process in terms of kJ/mol of reactant?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
The correct approach is “-2.67 kJ/mole“. A further solution is described below.
Explanation:
The given values are:
No. of moles,
= 5.60 mol
Substance releases,
ΔH = 14.9 kJ
Now,
The ΔH in terms of kJ/mol will be:
=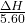
On putting the given values of ΔH, we get
=
=