Share
When maganese dioxide is added to hydrogen chloride you get water maganese dichloride and chlorine gas write the balanved equation
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: When maganese dioxide is added to hydrogen chloride you get water maganese dichloride and chlorine gas then balanced equation is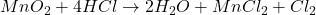 .
.
Explanation:
The word equation is given as maganese dioxide is added to hydrogen chloride you get water maganese dichloride and chlorine gas.
Now, in terms of chemical formulae this reaction equation will be as follows.
Here, number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
To balance this equation, multiply HCl by 4 on reactant side and multiply by 2 on product side. Therefore, the equation can be rewritten as follows.
by 2 on product side. Therefore, the equation can be rewritten as follows.
Hence, number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
Since, this equation contains same number of atoms on both reactant and product side. Therefore, this equation is now balanced equation.
Thus, we can conclude that when maganese dioxide is added to hydrogen chloride you get water maganese dichloride and chlorine gas then balanced equation is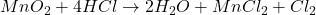 .
.