Share
Suppose a speck of dust in an electrostatic precipitator has 1×1012 protons in it and has a net charge of −5.2 nC (a very large charge for a
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Explanation:
The given data is as follows.
charge on an electron (e) =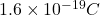
Formula to calculate number of electrons is as follows.
Q = ne
or, n =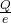
Putting the given values into the above formula as follows.
n =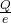
=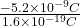
=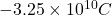
We know that when total charge is zero then number of protons is equal to the number of electrons.
That is,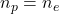
So, total number of electrons present are as follows.
=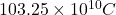
Therefore, we can conclude that given speck of dust has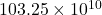 electrons.
electrons.