Share
Which subatomic particle has a negative charge? A) electron B) neutron C) nucleus D) proton
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
A) electron
Explanation:
Matter consists of atoms. Each atom consists of three particles:
– The proton: the protons are located inside the nucleus of the atom. They have a mass of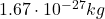 , and they have positive electric charge (
, and they have positive electric charge (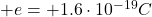 ). Protons are not fundamental particles, but they actually consists of three quarks (which are instead fundamental particles), in particular of 2 up quarks and 1 down quark.
). Protons are not fundamental particles, but they actually consists of three quarks (which are instead fundamental particles), in particular of 2 up quarks and 1 down quark.
– The neutron: neutrons are also located inside the nucleus. They have no electric charge, and their mass is similar to that of the proton (slightly larger). Neutrons consist of three quarks as well, in particular of 2 down quarks and 1 up quark.
– The electron: electrons are located outside the nucleus. They have a mass much smaller than protons (about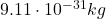 ) and they have a negative electric charge, opposite to that of the proton (
) and they have a negative electric charge, opposite to that of the proton (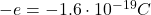 ). The electron is a fundamental particle.
). The electron is a fundamental particle.
Therefore, the subatomic particle having a negative charge is
A) electron