Share
What is the change in temperature, if a 70.0 gram sample of nickel absorbs 1756 J of energy? Nickel has a specific heat of 0.440 J/g °C.
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
Explanation:
Given that,
The mass of nickel, m = 70 g
Energy absorbed, Q = 1756 J
The specific heat of Nickel, c = 0.440 J/g °C
We know that,
The heat absorbed is given by :
So, the change in temperature is equal to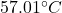 .
.