Share
The hydrogen ion concentration (h+) in a certain cleaning compound is (h+)=3.2×10^-11 use the formula ph=-log(h+) to find the pH of the clea
Question
The hydrogen ion concentration (h+) in a certain cleaning compound is (h+)=3.2×10^-11 use the formula ph=-log(h+) to find the pH of the clean compound the pH is approximately
in progress
0
Mathematics
3 years
2021-08-27T00:02:53+00:00
2021-08-27T00:02:53+00:00 1 Answers
10 views
0

Answers ( )
Answer:
The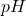 of the clean compound is approximately 10.495.
of the clean compound is approximately 10.495.
Step-by-step explanation:
If the cleaning ion concentration of the cleaning compound is![Rendered by QuickLaTeX.com [H^{+}] = 3.2\times 10^{-11}](https://documen.tv/wp-content/ql-cache/quicklatex.com-57becc0864dcf5d1a66517b875432d8c_l3.png) , then the
, then the 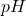 , no unit, of the compound is:
, no unit, of the compound is:
The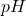 of the clean compound is approximately 10.495.
of the clean compound is approximately 10.495.