Share
Select the element(s) that will have ONE unpaired electron in the p orbital. Ca N B Ar
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: The element B will have ONE unpaired electron in the p orbital.
Explanation:
The electronic configuration of each given element is as follows.
Atomic number of calcium (Ca) is 20.
Ca: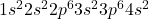
Atomic number of nitrogen (N) is 7.
N: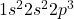
Atomic number of boron (B) is 5.
B: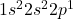
Atomic number of argon (Ar) is 18.
Ar: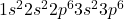
Atomic number of bromine (Br) is 35.
Br:![Rendered by QuickLaTeX.com [Ar] 4s^{2} 3d^{10} 4p^{5}](https://documen.tv/wp-content/ql-cache/quicklatex.com-5334657b1822936a431977c7200714ac_l3.png)
Therefore, boron is the only element that have one unpaired electron in the p-orbital.
Thus, we can conclude that element B will have ONE unpaired electron in the p orbital.