Share
One of the wavelengths of light emitted by hydrogen atoms under normal laboratory conditions is at ?0 = 656.3nm in the red portion of the el
Question
One of the wavelengths of light emitted by hydrogen atoms under normal laboratory conditions is at ?0 = 656.3nm in the red portion of the electromagnetic spectrum. In the light emitted from a distant galaxy this same spectral line is observed to be Doppler-shifted to ? = 953.3nm , in the infrared portion of the spectrum.
How fast are the emitting atoms moving relative to the earth?
in progress
0
Physics
3 years
2021-07-29T01:08:23+00:00
2021-07-29T01:08:23+00:00 1 Answers
18 views
0

Answers ( )
Answer:
1.07 × 10⁸ m/s
Explanation:
Using the relativistic Doppler shift formula which can be expressed as:
here;
Given that:
We will realize that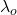 >
> 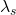 ; thus, v < 0 for this to be true.
; thus, v < 0 for this to be true.
From the above equation, let’s make (v/c) the subject of the formula: we have:
v = 0.357 c
To m/s:
1c = 299792458 m/s
∴
0.357c = (299 792 458 × 0.357) m/s
= 107025907.5 m/s
= 1.07 × 10⁸ m/s