Share
On another planet, the isotopes of titanium have the given natural abundances. Isotope Abundance Mass (u) 46Ti 77.600% 45.95263 48Ti 16.100%
Question
On another planet, the isotopes of titanium have the given natural abundances. Isotope Abundance Mass (u) 46Ti 77.600% 45.95263 48Ti 16.100% 47.94795 50Ti 6.300% 49.94479 What is the average atomic mass of titanium on that planet?
in progress
0
Physics
3 years
2021-08-23T05:18:36+00:00
2021-08-23T05:18:36+00:00 1 Answers
18 views
0

Answers ( )
Answer: Average atomic mass of titanium on that planet is 46.52
Explanation:
Mass of isotope Ti-46 = 45.95263
% abundance of isotope Ti-46 = 77.600 % =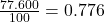
Mass of isotope Ti- 48= 47.94795
% abundance of isotope Ti-48 = 16.100%=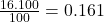
Mass of isotope Ti- 50 = 49.94479
% abundance of isotope Ti-50 = 6.300%=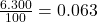
Formula used for average atomic mass of an element :
Average atomic mass of titanium on that planet is 46.52