Share
Identify the calculations possible using only 28.02 g/mol as a conversion factor. Select one or more:
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
The question is incomplete, the complete question is:
Identify the calculations possible using only 28.02 g/mol as a conversion factor. Select one or more:
(a): Calculate the grams of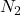 in 10.58 L of nitrogen gas
in 10.58 L of nitrogen gas
(b): Calculate the grams of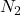 in
in 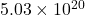 moles of nitrogen gas
moles of nitrogen gas
(c): Calculate the moles of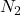 molecules in 3.94 grams of nitrogen gas
molecules in 3.94 grams of nitrogen gas
(d): Calculate the moles of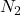 molecules in 4.73 L of nitrogen gas
molecules in 4.73 L of nitrogen gas
Answer: The correct options are (b) and (c).
Explanation:
We are given:
Molar mass of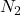 = 28.02 g/mol
= 28.02 g/mol
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
At STP conditions:
1 mole of gas occupies 22.4 L of volume
For the given options:
(a): Volume is given and to calculate the mass of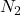 , we need to use both the conversion factors above.
, we need to use both the conversion factors above.
The equation formed will be:
Mass of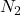 =
= 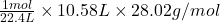
(b): Moles are given and to calculate the mass of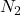 , we need only the first conversion factor.
, we need only the first conversion factor.
The equation formed will be:
Mass of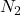 =
= 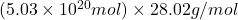
(c): Mass is given and to calculate the moles of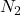 molecules, we need only the first conversion factor.
molecules, we need only the first conversion factor.
The equation formed will be:
Moles of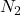 molecules =
molecules = 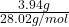
(d): Volume is given and to calculate the moles of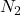 molecules, we need only the second conversion factor.
molecules, we need only the second conversion factor.
The equation formed will be:
Moles of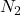 molecules =
molecules = 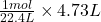
Hence, the correct option is (b) and (c)