Share
From what height must an oxygen molecule fall in a vacuum so that its kinetic energy at the bottom equals the average energy of an oxygen mo
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
The value is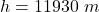
Explanation:
From the question we are told that
The temperature is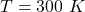
Generally the root mean square speed of the oxygen molecules is mathematically represented as
Here R is the gas constant with a value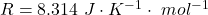
M is the molar mass of oxygen molecule with value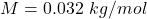
So
=>