Share
Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas. How many grams of oxygen are formed when 5.00 g of dinitrogen monoxi
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
Mass of oxygen are formed is = 1.808 gm
Explanation:
Reaction equation of given condition
No. of moles of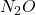
m = 5 gm & M = 44 gm
N = 0.113 mol
Now no. of moles of oxygen formed =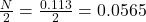
Now mass of oxygen formed is
m = N M
m = 0.0565 × 32
m = 1.808 gm
Thus mass of oxygen are formed is = 1.808 gm