Share
Consider the reaction described by the following chemical equation. 2 HN3(1) + 2 NO(g) — H2O2(1) + 4 N, (g) What is the enthalpy
Question
Consider the reaction described by the following chemical equation.
2 HN3(1) + 2 NO(g) — H2O2(1) + 4 N, (g)
What is the enthalpy change associated with the production of 1 mol of H, O, if a reaction that produces
2.50 g of H, O, releases 65.9 kJ of heat?
in progress
0
Chemistry
3 years
2021-08-30T13:38:15+00:00
2021-08-30T13:38:15+00:00 1 Answers
10 views
0

Answers ( )
Answer:
Q = -897 kJ/mol
Explanation:
From the given information:
The heat released Q = -65.9 kJ
To start with the molar mass of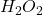 = 2 × (molar mass of H) + 2 × (molar mass of O)
= 2 × (molar mass of H) + 2 × (molar mass of O)
= (2 × 1.008) + (2 × 16.0 )
= 34.016 g/mol
However, given that:
mass of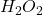 2.50 g
2.50 g
The number of moles of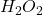 =
= 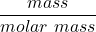
Finally; Using the formula:
Q = -897 kJ/mol