Share
Consider the reaction, C2H4(g) + H2(g) – C2H6(8), where AH = -137 kJ. How many kilojoules are released when 3.5 mol of CH4 reacts?
Question
Consider the reaction, C2H4(g) + H2(g) – C2H6(8), where AH = -137 kJ. How many kilojoules are released when 3.5 mol of CH4
reacts?
480 kJ are released
20 x 103 kJ are released
570 kJ are released
137 kJ are released
in progress
0
Chemistry
3 years
2021-09-05T07:44:00+00:00
2021-09-05T07:44:00+00:00 1 Answers
54 views
0

Answers ( )
Answer: 480 kJ of energy is released when 3.5 mol of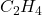 reacts.
reacts.
Explanation:
The balanced chemical reaction is:
Thus it is given that the reaction is exothermic (heat energy is released) as enthalpy change for the reaction is negative.
1 mole of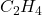 on reacting gives = 137 kJ of energy
on reacting gives = 137 kJ of energy
Thus 3.5 moles of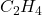 on reacting gives =
on reacting gives = 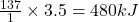 of energy
of energy
Thus 480 kJ of energy is released when 3.5 mol of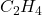 reacts.
reacts.