Share
Atmospheric pressure is reported in a variety of units depending on local meteorological preferences. In many European countries the unit mi
Question
Atmospheric pressure is reported in a variety of units depending on local meteorological preferences. In many European countries the unit millibar (mbar) is preferred, in other countries the unit hectopascal (1 hPa = 1 mbar) is used, and in the United States inches of mercury (in Hg) is the commonly used unit. In most chemistry textbooks the units most commonly used are torr, mmHg, and atmospheres (atm). The unit atm is defined at sea level to be 1 atm = 760 mm Hg exactly. The density of mercury is 13.534 times that of water, if atmospheric pressure will support 769.6 mm Hg, what height of a water column would that same pressure support in mm?
in progress
0
Physics
3 years
2021-09-04T15:55:37+00:00
2021-09-04T15:55:37+00:00 1 Answers
7 views
0

Answers ( )
Answer:
Explanation:
Given:
density of mercury,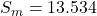
height of the mercury column supported by the atmosphere,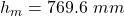
As we know that the equivalent pressure in terms of liquid column is given as:
so,
where: