Share
At what temperature will uranium hexafluoride , the densest gas known have the same average speed as a molecule of the lightest gas, hydroge
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
the required temperature of uranium hexafluoride is 54156.25 K
Explanation:
Given the data in the question;
We know that average speed is;
u =![Rendered by QuickLaTeX.com [ 3RT / MM ]^{1/2](https://documen.tv/wp-content/ql-cache/quicklatex.com-17933c7d8aa0653ed9de4801c8d5f3e4_l3.png) ———– let this be equation 1
———– let this be equation 1
where MM is the molar mass
T is temperature
R is universal gas constant and u is the average speed.
First we get the average speed of H₂
Next is the average speed of UF₆
given that; both have the same average speed, equation 2 = equation 3;
we multiply both sides by 1/3R and also square both sides.
given that; temperature of hydrogen T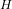 ₂ = 37°C = ( 37 + 273.15)K = 310.15 K
₂ = 37°C = ( 37 + 273.15)K = 310.15 K
we know that Molar mass of H₂; MM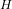 ₂ = 2.016 g/mol
₂ = 2.016 g/mol
and molar mass of UF₆; MM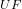 ₆ = 352.02 g/mol
₆ = 352.02 g/mol
so we substitute
[ 310.15 K / 2.016 g/mol ] = [ T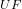 ₆ / 352.02 g/mol ]
₆ / 352.02 g/mol ]
T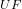 ₆ = [ 352.02 g/mol × 310.15 K ] / 2.016 g/mol
₆ = [ 352.02 g/mol × 310.15 K ] / 2.016 g/mol
T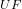 ₆ = 109179.003 K/ 2.016
₆ = 109179.003 K/ 2.016
T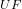 ₆ = 54156.25 K
₆ = 54156.25 K
Therefore, the required temperature of uranium hexafluoride is 54156.25 K