Share
An electron has a kinetic energy of 10.1 eV. The electron is incident upon a rectangular barrier of height 18.2 eV and width 1.00 nm. If the
Question
An electron has a kinetic energy of 10.1 eV. The electron is incident upon a rectangular barrier of height 18.2 eV and width 1.00 nm. If the electron absorbed all the energy of a photon of green light (with wavelength 546 nm) at the instant it reached the barrier, by what factor would the electron’s probability of tunneling through the barrier increase
in progress
0
Physics
3 years
2021-08-24T02:19:25+00:00
2021-08-24T02:19:25+00:00 1 Answers
3 views
0

Answers ( )
Answer:
factor that the electron’s probability of tunneling through the barrier increase 2.02029
Explanation:
given data
kinetic energy = 10.1 eV
height = 18.2 eV
width = 1.00 nm
wavelength = 546 nm
solution
we know that probability of tunneling is express as
probability of tunneling =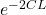 ……………..1
……………..1
here C is =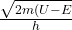
here h is Planck’s constant
c =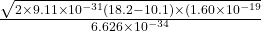
c = 2319130863.06
and proton have hf =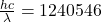 = 2.27 ev
= 2.27 ev
so electron K.E = 10.1 + 2.27
KE = 12.37 eV
so decay coefficient inside barrier is
c’ =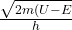
c’ =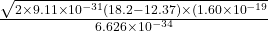
c’ = 1967510340
so
the factor of incerease in transmisson probability is
probability =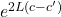
probability =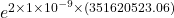
factor probability = 2.02029