Share
Air exits a turbine at 200 kPa and 1508C with a volumetric flow rate of 7000 liters/s. Modeling air as an ideal gas, determine the mass flow
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
mass flow rate = 11.464 kg/sec
Explanation:
given data
P = 200 kPa = 2 × Pa
Pa
temperature = 150°C = 423 k
volumetric flow rate = 7000 liters/s
solution
first we get here molar flow rate that is express as
molar flow rate =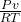 ……………..1
……………..1
put here value
molar flow rate =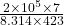
molar flow rate = 398.06 mol/sec
and
now we get mass flow rate
mass flow rate = molar flow rate × average mol weight …………..2
mass flow rate = 398.06 × 28.8
mass flow rate = 11464.3 g/sec
mass flow rate = 11.464 kg/sec