Share
According to the reaction, below, how many grams of aluminum are needed to react fully with 100 grams of sulfur? 2AL + 3s – Al2S3
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: 56.2 g of Aluminium is needed to react fully with 100 grams of sulphur.
Explanation:
To calculate the moles :
The balanced chemical equation is:
According to stoichiometry :
3 moles of require = 2 moles of
require = 2 moles of 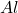
Thus 3.125 moles of will require=
will require= of
of 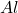
Mass of
Thus 56.2 g of Aluminium is needed to react fully with 100 grams of sulphur.