Share
A sample of gas has a volume of 0.600 L at a temperature of 30.oC and a pressure of 0.800 atm. What is the number of moles in this sample
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
0.00019 moles
Explanation:
Use the Ideal Gas Law formula: PV=nRT
Remember:
P = pressure (atm)
V = volume (L)
n = number of moles (mol)
R = molar gas constant (8.314 J*mol/K)
T = Temperature (K)
1. First rearrange the formula so that it would be easier to find the number of moles.
n=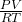
2. Convert the Temperature from Celcius to Kelvin (remember the temperature is always used in Kelvin for the formula).
30° C + 273 K = 303 K
3. Now substitude your values
n=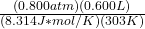
4. Find your value
n=0.0019 mol