Share
A mixture of nitrogen and argon gas is expanded from a volume of to a volume of 62 L to 84 L, while the pressure is held constant at 96 atm
Question
A mixture of nitrogen and argon gas is expanded from a volume of to a volume of 62 L to 84 L, while the pressure is held constant at 96 atm . Calculate the work done on the gas mixture. Round your answer to significant digits, and be sure it has the correct sign (positive or negative).
in progress
0
Physics
4 years
2021-08-25T10:59:38+00:00
2021-08-25T10:59:38+00:00 1 Answers
9 views
0

Answers ( )
Explanation:
The given data is as follows.
P = 96 atm,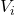 = 62 L
= 62 L
Now, we know that the relation between work and pressure is as follows.
W =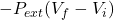
Putting the given values into the above formula we will calculate the work done as follows.
W =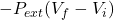
=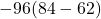
= -2112 J
or, = -211.2 kJ (as 1 kJ = 1000 J)
Thus, we can conclude that the work done on the gas mixture is 211.2 kJ.