Share
A Carnot heat engine uses a hot reservoir consisting of a large amount of boiling water and a cold reservoir consisting of a large tub of ic
Question
A Carnot heat engine uses a hot reservoir consisting of a large amount of boiling water and a cold reservoir consisting of a large tub of ice and water. In 5 minutes of operation of the engine, the heat rejected by the engine melts a mass of ice equal to 3.30×10−2 kg . Throughout this problem use Lf=3.34×105J/kg for the heat of fusion for water.A) During this time, how much work W is performed by the engine?
in progress
0
Physics
3 years
2021-08-30T16:47:03+00:00
2021-08-30T16:47:03+00:00 1 Answers
10 views
0

Answers ( )
Answer:
Explanation:
Given:
mass of ice melted,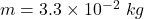
time taken by the ice to melt,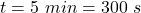
latent heat of the ice,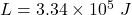
Now the heat rejected by the Carnot engine:
Since we have boiling water as hot reservoir so:
The cold reservoir is ice, so:
Now the efficiency:
Now form the law of energy conservation:
Heat supplied:
where:
Now the work done: