Share
A 72.4 mL solution of Cu(OH) is neutralized by 47.8 mL of a 0.56 M H2(C204) solution. What is the concentration of the Cu(OH)?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: The concentration of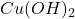 is 0.369 M.
is 0.369 M.
Explanation:
Given: = 72.4 mL,
= 72.4 mL, 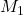 = ?
= ?
Formula used to calculate the concentration of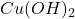 is as follows.
is as follows.
Substitute the values into above formula as follows.
Thus, we can conclude that the concentration of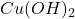 is 0.369 M.
is 0.369 M.