Share
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is establish
Question
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Assuming the surroundings to be at 20°C, determine the amount of work that could have been produced if the entire process were executed in a reversible manner.
in progress
0
Physics
3 years
2021-08-28T17:24:45+00:00
2021-08-28T17:24:45+00:00 1 Answers
440 views
0

Answers ( )
Answer:
The amount of work produced by the entire process of cooling the iron and the copper is 1969.5 kJ
Explanation:
Given;
mass of iron = 50 kg
mass of copper = 20 kg
initial temperature of iron and copper = 80 °C
temperature of the lake = 15°C
Both iron and copper forms a closed system and attained thermal equilibrium.
This energy balance can be expressed as;
Net heat transfer = change in internal energy
Δ = Δ
= Δ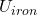 + Δ
+ Δ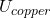
And U = McΔT
C is specific heat capacity; ( iron = 0.45 kJ/kg.K, copper = 0.39 kJ/kg.K)
ΔU = 50 x 0.45 (80 -15) + 20 x 0.39 (80 -15)
ΔU = 1462.5 kJ + 507 kJ = 1969.5 kJ
The amount of work produced by the entire process of cooling the iron and the copper is 1969.5 kJ