Share
Nitrogen at an initial state of 300 K, 150 kPa, and 0.2 m3is compressed slowly in an isothermal process to a final pressure of 800 kPa. Dete
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
Explanation:
Given:
Isothermal process.
initial temperature,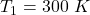
initial pressure,
initial volume,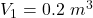
final pressure,
The work done during an isothermal process is given by: