Share
For each carbon-14 atom, there are approximately 10^12 carbon-12 atoms. This ratio is constant in each organism. The carbon-12 remains const
Question
For each carbon-14 atom, there are approximately 10^12 carbon-12 atoms. This ratio is constant in each organism. The carbon-12 remains constant but the carbon-14 decays. Suppose a piece of wood is analysed, and it contains 10^14 carbon-12 atoms and 40 carbon-14 atoms.
1) Determine how many carbon-14 atoms were present in the wood when it died.
2) Use the half life of carbon-14 atoms to obtain a function to model the number N of carbon-14 atoms present in the wood (t) years after it died
in progress
0
Mathematics
3 years
2021-08-14T16:26:03+00:00
2021-08-14T16:26:03+00:00 1 Answers
6 views
0

Answers ( )
Answer:
1) There are 100 carbon-14 atoms in the wood when it died
2) A function that models the number ‘N’ of carbon-14 atoms present in the wood (t) years after it died is presented as follows;
Step-by-step explanation:
The given parameters are;
The number of carbon-12 for each carbon-14 atom ≈ 10¹² carbon-12 atoms
The number of carbon-12 in the piece of wood = 10¹⁴ carbon-12 atoms
The number of carbon-14 in the piece of wood = 40 carbon-14 atoms
The number of carbon-12 in an organism = Constant
The number of carbon-14 in an organism = Decays
1) Given that the number of carbon-12 in an organism is constant, and there are 10¹² carbon-12 atoms per each carbon-14 atom, therefore, we have;
The number of carbon-12 atoms in the wood when it died = 10¹⁴ carbon-12 atoms
The number of carbon-14 atoms in the wood when it died = (10¹⁴ carbon-12 atoms)/(10¹² carbon-12 atoms/(carbon-14 atom)) = 100 carbon-14 atoms
The number of carbon-14 atoms in the wood when it died = 100 carbon-14 atoms
2) The half life of a radioactive isotope is given by the following formula;
The half life of carbon-14 atoms,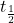 ≈ 5,730 years
≈ 5,730 years
N₀ = The amount of carbon-14 present in the wood when it died = 100 carbon-14 atoms
Therefore, we have;
The number, N, of carbon-14 atoms present in the wood (t) years after it died is given as follows;