Share
At the same temperature and pressure, 15L of CO2 gas has the same amount of molecules as what volume of N2 gas?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: 15 L of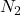 gas will have same numer of molecules as 15L of
gas will have same numer of molecules as 15L of 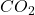 gas at the same temperature and pressure,
gas at the same temperature and pressure,
Explanation:
According to avogadro’s law, equal moles of gases occupy equal volume at same conditions of temperature and pressure.
Thus 0.67 moles of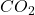 contains=
contains=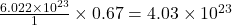 molecules
molecules
Now molecules of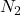 = molecules of
= molecules of 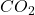 =
= 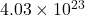
Now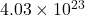 molecules of
molecules of 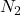 =
=