Share
An oxygen molecule is adsorbed onto a small patch of the surface of a catalyst. It’s known that the molecule is adsorbed on of possible site
Question
An oxygen molecule is adsorbed onto a small patch of the surface of a catalyst. It’s known that the molecule is adsorbed on of possible sites for adsorption (see sketch at right). Calculate the entropy of this system. Round your answer to significant digits, and be sure it has the correct unit symbol.
in progress
0
Physics
3 years
2021-09-03T09:40:58+00:00
2021-09-03T09:40:58+00:00 1 Answers
0 views
0

Answers ( )
The given question is incomplete. The complete question is as follows.
An oxygen molecule is adsorbed onto a small patch of the surface of a catalyst. It’s known that the molecule is adsorbed on 1 of 36 possible sites for adsorption. Calculate the entropy of this system.
Explanation:
It is known that Boltzmann formula of entropy is as follows.
s = k ln W
where, k = Boltzmann constant
W = number of energetically equivalent possible microstates or configuration of the system
In the given case, W = 36. Now, we will put the given values into the above formula as follows.
s = k ln W
=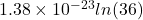
=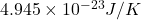
Thus, we can conclude that the entropy of this system is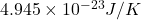 .
.