Share
a bag of ice cubes absorbs 149,000 J of heat, which causes its temperature to increase by 5.23 degrees celsius. what is the mass of the ice
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: 14.2447
Explanation:
Answer:
14.3kg
Explanation:
Given parameters:
Quantity of heat = 149000J
Change in temperature = 5.23°C
specific heat of the ice = 2000J/kg°C
Unknown:
Mass of the ice in the bag = ?
Solution:
The heat capacity of a substance is given as:
H = m c Ф
H is the heat capacity
m is the mass
c is the specific heat
Ф is the temperature change;
since m is the unknown, we make it the subject of the expression;
m = H/ mФ
m =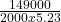 = 14.3kg
= 14.3kg