Share
A 72.0 g sample of an organic solid is dissolved in 180mL of water. The solid is extracted using one 60 mL extraction in the first extractio
Question
A 72.0 g sample of an organic solid is dissolved in 180mL of water. The solid is extracted using one 60 mL extraction in the first extraction of an organic solvent which has a partition (distribution) coefficient with water of 10. The first extraction removed 55.4 g of solid from water. What are the numbers that need to go in box A and B to calculate the volume of solvent (y) that would be necessary to remove an additional 7.0g from the remaining sample dissolved in water. You DON’T have to complete the calculation to solve for y.
in progress
0
Chemistry
3 years
2021-09-05T08:54:19+00:00
2021-09-05T08:54:19+00:00 1 Answers
0 views
0

Answers ( )
Answer:
Explanation:
From the question we are told that
mass of sample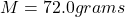
volume of water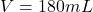
volume for extraction
partition (distribution) coefficient water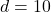
initial extraction removal
Generally the equation for the weight of sample is mathematically given by
is mathematically given by
Generally the weight extracted is therefore
is therefore
Therefore volume of solvent (y) that would be necessary to remove an additional 7.0g