Share
The specific heat of water is 4.184Jg ∘C. Determine the final temperature when 600.0 g water at 75.5∘C absorbs 5.90×104 J of energy.]
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
Explanation:
Given that,
The specific heat of water is 4.184Jg°C
Mass, m = 600 g
Initial temperature, T₁ = 75.5°C
We need to find the final temperature. We know that heat absorbed is given by :
So, the final temperature is equal to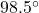 .
.