Share
The gas in a balloon has T=280K and V=0.0279m^3. If the temperature increases to 320K at constant pressure, what is the new volume of the ba
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
Explanation:
According to Charles Law
=>
Where = 0.0279 m³,
= 0.0279 m³,  = 280 K and
= 280 K and 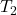 = 320 K
= 320 K
=>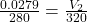
=> = 0.03189 m³
= 0.03189 m³