Share
What is the concentration of a solution with a volume of 3.3 mL that contains 12 g of ammonium sulfite? (molar mass = 100.154 g/mol)
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
36.30 M
Explanation:
Given that:
Molar mass of ammonium sulfite (NH₄)₂ SO₃ = 100.154 g/mol
Volume = 3.3 mL
to liters, we have: (3.3/1000)L
= 0.0033 L
mass = 12 grams
The concentration of the solution =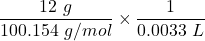
= 36.30 M