Share
The solution you identified in question (1) acts as a buffer due to reactions that occur within the solution when an acid or a base is added
Question
The solution you identified in question (1) acts as a buffer due to reactions that occur within the solution when an acid or a base is added. Write the net ionic chemical equation for the reaction that occurs within this buffer solution when HCl(aq) is added. (Phase labels should be included in all net ionic chemical equations.)
in progress
0
Chemistry
4 years
2021-08-24T14:46:38+00:00
2021-08-24T14:46:38+00:00 1 Answers
17 views
0

Answers ( )
Answer:
The answer is “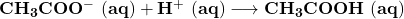 “
“
Explanation:
When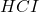 is added in the chemical equation it reacts with sodium acetate so, it will give the following chemical equation:
is added in the chemical equation it reacts with sodium acetate so, it will give the following chemical equation:
In this, the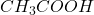 is a weak acid so, it not completely dissociated.
is a weak acid so, it not completely dissociated.
The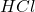 is a strong acid so, it is completely dissociated So, the net ionic equation is:
is a strong acid so, it is completely dissociated So, the net ionic equation is: