Share
The heating element in an electric kettle is rated as 2.0 kW. If the water in the kettle is at 100.0 °C, what volume of water will be conve
Question
The heating element in an electric kettle is rated as 2.0 kW. If the water in the kettle is at 100.0 °C, what volume of water will be converted into steam in one minute? The specific latent heat of vaporization of the water is 2,257,000 J/kg and the
3 density of water is 1,000 kg/m .
in progress
0
Physics
5 years
2021-09-03T08:58:27+00:00
2021-09-03T08:58:27+00:00 1 Answers
22 views
0

Answers ( )
Answer:
The volume is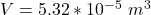
Explanation:
From the question we are told that
The power of the heating element is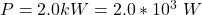
The temperature of the water in the kettle is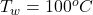
The time to convert water to steam is t = 1 minute = 60 sec
The specific latent heat of vaporization is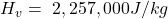
The density of water is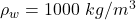
The power of the heating element is mathematically represented as
Where E Energy generated by the heating element in term of heat
substituting values
Now
The latent heat of vaporization is mathematically represented as
Where m is the mass of water converted to steam
So
substituting values
The volume of water converted to steam is mathematically evaluated as
substituting values