Share
SOMEONE HELP ME 1. How many grams of C are present in 4.86 grams of carbon dioxide ? grams C. 2. How many grams of c
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
1. 1.33 gram of carbon
2. 2.38g of carbon dioxide
Explanation:
From the given information:
Total amount of CO₂ = 4.86 grams
Atomic mass of C = 12 g/mole
molar mass of CO₂ = 44 g/mole
∴
The mass of the Carbon (C) in grams is:
= 1.33 gram of carbon
2.
Here, the total amount of CO₂ = unknown
Atomic mass of O₂ = 32 g/mole
molar mass of CO₂ = 44 g/mole
amount of oxygen = 1.73 g
∴
The mass of CO₂ =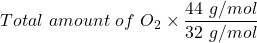
= 2.38 g of carbon dioxide