Share
In science, we like to develop explanations that we can use to predict the outcome of events and phenomena. Try to develop an explanation th
Question
In science, we like to develop explanations that we can use to predict the outcome of events and phenomena. Try to develop an explanation that tells how much NaOH needs to be added to a beaker of HCl to cause the color to change. Your explanation can be something like: g
in progress
0
Chemistry
5 years
2021-08-12T13:52:37+00:00
2021-08-12T13:52:37+00:00 1 Answers
49 views
0

Answers ( )
The question is incomplete. The complete question is :
In science, we like to develop explanations that we can use to predict the outcome of events and phenomena. Try to develop an explanation that tells how much NaOH needs to be added to a beaker of HCl to cause the color to change. Your explanation can be something like: The color change will occur when [some amount] of NaOH is added because the color change occurs when [some condition]. The goal for your explanation is that it describes the outcome of this example, but can also be used to predict the outcome of other examples of this phenomenon. Here’s an example explanation: The color of the solution will change when 40 ml of NaOH is added to a beaker of HCl because the color always changes when 40ml of base is added. Although this explanation works for this example, it probably won’t work in examples where the flask contains a different amount of HCl, such as 30ml. Try to make an explanation that accurately predicts the outcome of other versions of this phenomenon.
Solution :
Consider the equation of the reaction between NaOH and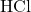
NaOH (aq) + HCl (aq) → NaCl(aq) +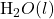
The above equation tells us that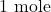 of
of 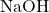 reacts with
reacts with 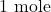 of
of 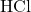 .
.
So at the equivalence point, the moles of NaOH added = moles of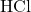 present.
present.
If the volume of the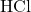 taken =
taken = 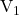 mL and the conc. of
mL and the conc. of 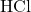 =
= 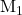 mole/L
mole/L
The volume of NaOH added up to the color change =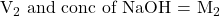 mole/L
mole/L
Moles of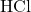 taken =
taken = 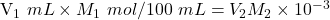 moles.
moles.
The color change will occur when the moles of NaOH added is equal to the moles of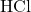 taken.
taken.
Thus when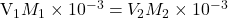
or when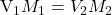
or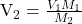 mL of NaOH added, we observe the color change.
mL of NaOH added, we observe the color change.
Where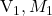 are the volume and molarity of the
are the volume and molarity of the 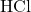 taken.
taken.
When both the NaOH and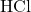 are of the same concentrations, i.e. if
are of the same concentrations, i.e. if 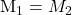 , then
, then 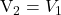
Or the 40 mL of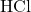 will need 40 mL of NaOH for a color change and
will need 40 mL of NaOH for a color change and
30 mL of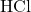 would need 30 mL of NaOH for the color change (provided the concentration
would need 30 mL of NaOH for the color change (provided the concentration 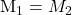 )
)