How many calories are released when 6 grams of 100°C steam turns to 0°C ice? Question How many calories are released when 6 grams of 100°C steam turns to 0°C ice? in progress 0 Physics RI SƠ 4 years 2021-09-02T18:05:52+00:00 2021-09-02T18:05:52+00:00 1 Answers 12 views 0
Answers ( )
Answer: 4276.2 calories
Explanation:
Given
mass of steam is 6 gm at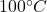
Conversion of steam to ice involves
Calories released during the conversion of steam to water at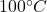
Calories released during the conversion of water at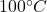 to water at
to water at 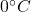
Calories released during the conversion of water to the ice at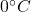
The total energy released is