Share
Calculate the amount of heat required to completely sublime 57.0 g of solid dry ice (CO2) at its sublimation temperature. The heat of sublim
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
41.48KJ
Explanation:
the amount of heat(q)=nΔ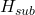
n represents the number of moles of the substance
n=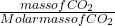
mass of carbondioxide = 57g
the molar mass of carbondioxide is obtained by adding the atomic mass of the individual elements present
atomic mass of carbon = 12g
atomic mass for oxygen = 16 × 2 =32g
Molar mass of carbondioxide = 12 + 32 = 44g/mole
∴ n = 57÷44
n = 1.2955moles
Δ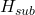 represents the heat of sublimation for the substance in that sublimation process = 32.3kJ/mol
represents the heat of sublimation for the substance in that sublimation process = 32.3kJ/mol
the amount of heat (q) = 1.2955 × 32.3
q= 41.84KJ
the amount of heat required to completely sublime 57g of carbondioxide is 41.48KJ
Answer:
41.83 J.
Explanation:
The amount of heat required to completely sublime CO₂ is
Q = cm’ ……………… Equation 1
Where Q = Amount of heat required to sublime CO₂, c = heat of sublimation of CO₂, m’ = number of moles of CO₂.
Molar mass of CO₂ = 12 + (16×2) = 12×32 = 44 g/mole,
If 1 mole of CO₂ contains a mass of 44 g,
57/44 moles of CO₂ will contain a mass of 57 g.
1.295 moles.
Given: c = 32.3 kJ/mol, n = 1.295 mol.
Substitute into equation 1
Q = 32.3(1.295)
Q = 41.83 J.