Share
A 41 g Ice cube at -21 C is dropped into a container of water at 0 C. How much water freezes onto the ice? The specific heat of ice is .5 ca
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
The right solution is “5.38 grams“.
Explanation:
The given values are:
Heat of fusion,
L = 80 cal/g
Mass of ice cube,
Specific heat of ice,
Let,
Gram of water freezes will be “m”.
⇒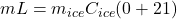
Or,
⇒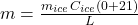
On substituting the values, we get
⇒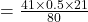
⇒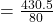
⇒