Share
A 3mL sample of a 200 Molar solution is left open on a lab counter for two weeks, after which the concentration of the solution is 6 M
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: New volume of the solution is 100 mL.
Explanation:
Given: = 3 mL,
= 3 mL, 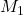 = 200 M
= 200 M
Formula used to calculate the new volume of solution is as follows.
Substitute the values into above formula as follows.
Thus, we can conclude that new volume of the solution is 100 mL.