Share
17) A 0.20 M solution of HCl with a volume of 15.0 mL is exactly neutralized by the 0.10 M solution of NaOH with what volume? HINT: Use the
Question
17) A 0.20 M solution of HCl with a volume of 15.0 mL is exactly neutralized by the 0.10 M solution of NaOH with what volume? HINT: Use the titration formula from table T. First multiply, then divide. o 1.5 mL O 7.5 mL 7 o 3.0 mL 0 30. mL
in progress
0
Chemistry
4 years
2021-07-17T02:42:16+00:00
2021-07-17T02:42:16+00:00 1 Answers
15 views
0

Answers ( )
Answer: A 0.20 M solution of HCl with a volume of 15.0 mL is exactly neutralized by the 0.10 M solution of NaOH with 3 mL volume.
Explanation:
Given: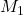 = 0.20 M,
= 0.20 M,  = 15.0 mL
= 15.0 mL
Formula used is as follows.
Substitute the values into above formula s follows.
Thus, we can conclude that a 0.20 M solution of HCl with a volume of 15.0 mL is exactly neutralized by the 0.10 M solution of NaOH with 3 mL volume.