Share
Q = cmAT 33. Use the formula to show that 3000 cal are required to raise the temperature of 300 g of water from 20°C to 30
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: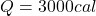
Explanation:
We are given the following formula:
Where:
Rewriting equation (1) with the known values at the right side, we will prove the result is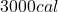 :
: