Share
Please Help!! Please, this is due in a couple of minutes, please help If 8.51×10-2 moles of SO3(g), 0.210 moles of SO2, and 0.35
Question
Please Help!! Please, this is due in a couple of minutes, please help
If 8.51×10-2 moles of SO3(g), 0.210 moles of SO2, and 0.354 moles of O2 are at equilibrium in a 15.4 L container at 1.25×103 K, the value of the equilibrium constant, K, is ____
in progress
0
Chemistry
4 years
2021-08-23T07:38:15+00:00
2021-08-23T07:38:15+00:00 1 Answers
9 views
0

Answers ( )
Answer: The value of equilibrium constant, K, is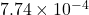 .
.
Explanation:
The reaction equation is as follows.
Now, the expression for equilibrium constant of this reaction is as follows.
Substitute the given values into above expression as follows.
Thus, we can conclude that the value of equilibrium constant, K, is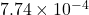 .
.