Share
Liquid chlorobenzene goes through a process where there is negligible heat flow and no work done on the surroundings. initially it is at 330
Question
Liquid chlorobenzene goes through a process where there is negligible heat flow and no work done on the surroundings. initially it is at 330 k and 3,500 kpa. at the end of the process the final pressure is 2,000kpa. estimate the temperature change and the entropy change of chlorobenzene.
in progress
0
Physics
4 years
2021-08-10T20:26:47+00:00
2021-08-10T20:26:47+00:00 1 Answers
26 views
0

Answers ( )
Answer:
The change in temperature is -141.428K
The entropy change is -60.75J/mole K Kg
Explanation:
Given that,
Initial Temperature, T₁ = 330k
Initial Pressure, P₁ = 3500kpa
Final pressure, P₂ = 2000kpa
Since the work done on the surrounding is zero
hence, the change in volume of the system will be zero and the process will be constant
Calculate the Temperature of the system
Substitute the value in the above equation
Calculate the change in temperature
ΔT = T₂ – T₁
= 188.57K – 330K
= -141.428K
The change in temperature is -141.428K
Expression of change in entropy
ds is the entropy change
equation for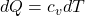
Substitute for dQ in the expression of change in entropy and integrate
substitute the values
The entropy change is -60.75J/mole K Kg