Share
It is well-known that carbon dioxide, CO2, has a much greater density than air. In fact, CO2 gas can displace air, which is why there
Question
It is well-known that carbon dioxide, CO2, has a much greater density than air. In fact,
CO2 gas can displace air, which is why there are regulations in place that limit the
amount of dry ice allowed in elevators. In other words, do not get trapped in an
elevator, or any enclosed space, with someone who is transporting dry ice. Calculate
the pressure exerted by the CO2 gas, in atm, if the density was measured to be 1.983
g/L on a day where the temperature is 22.165 °C.
in progress
0
Chemistry
4 years
2021-08-10T21:14:28+00:00
2021-08-10T21:14:28+00:00 1 Answers
14 views
0

Answers ( )
Answer: The pressure exerted by the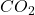 gas, in atm is 1.092
gas, in atm is 1.092
Explanation:
According to the ideal gas equation:’
P = Pressure of the gas = ?
V= Volume of the gas
T= Temperature of the gas = (0°C = 273 K)
(0°C = 273 K)
n= moles of gas =
R= Value of gas constant = 0.0821 Latm/K mol
Thus pressure exerted by the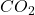 gas, in atm is 1.092
gas, in atm is 1.092