Share
In this experiment, you will need to prepare 250.0 mL of 0.100 M KCl(aq) solution. Determine the mass in grams of potassium chloride require
Question
In this experiment, you will need to prepare 250.0 mL of 0.100 M KCl(aq) solution. Determine the mass in grams of potassium chloride required to prepare this solution. Type your numerical answer (no units) in the box below. Even though the number of significant figures is limited to three by the inputs, please report your answer to four significant figures.
in progress
0
Chemistry
4 years
2021-08-15T23:58:45+00:00
2021-08-15T23:58:45+00:00 1 Answers
25 views
1

Answers ( )
Answer: The mass in grams of potassium chloride required to prepare this solution is 1.862 grams
Explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
where,
n = moles of solute
moles of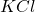 =
= 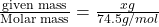
Now put all the given values in the formula of molality, we get
Therefore, the mass in grams of potassium chloride required to prepare this solution is 1.862 grams