Share
determine the number of moles and mass of the sample of argon occupying 37.8L at STP
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
The number of moles and mass of the sample of argon occupying 37.8L at STP are 1.6875 moles and 67.41 grams respectively.
Explanation:
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
Then you can apply the following rule of three: if by definition of STP 22.4 L are occupied by 1 mole of Ar, 37.8 L of Ar are occupied by how many moles?
amount of moles= 1.6875 moles
The atomic weight of Ar is 39.948 g/mol. So the mass of the sample of argon can be calculated as:
1.6875 moles* 39.948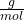 = 67.41 grams
= 67.41 grams
The number of moles and mass of the sample of argon occupying 37.8L at STP are 1.6875 moles and 67.41 grams respectively.