Share
Calculate the value of the translational partition function of O2 at 1000 K for a volume of 1 m3. Submit your answer multiplied by 10^−32 (i
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
The value of the function is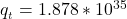
Explanation:
From the question we are told that
The temperature is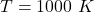
The volume is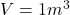
Generally the transnational partition function is mathematically represented as
Where m is the molar mass of oxygen with a constant value of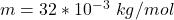
k is the Boltzmann constant with a value of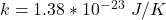
h is the Planck’s constant with value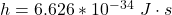
Substituting values