Share
Calculate the total energy, in kilojoules, that is needed to turn a 46 g block of ice at -25 degrees C into water vapor at 100 degrees
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: The total energy, in kilojoules, that is needed to turn a 46 g block of ice at -25 degrees C into water vapor at 100 degrees C is 11.787 kJ.
Explanation:
Given: Mass = 46 g
Initial temperature =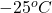
Final temperature =
Specific heat capacity of ice = 2.05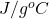
Formula used to calculate the energy is as follows.
where,
q = heat energy
m = mass
C = specific heat capacity
Substitute the values into above formula as follows.
Thus, we can conclude that the total energy, in kilojoules, that is needed to turn a 46 g block of ice at -25 degrees C into water vapor at 100 degrees C is 11.787 kJ.